Introduction
Polychlorinated biphenyls (PCBs), dioxins, furans, and polychlorinated dibenzo-p-dioxins (PCDDs) constitute a group of persistent organic pollutants with adverse health implications. These toxic compounds often evade the public eye but have garnered attention due to their presence and bioaccumulation in food chains, in particular marine oils.
Health Risks
PCBs: Polychlorinated biphenyls, initially employed in various industrial applications, are notorious for their long-lasting effects on the environment and human health. PCBs are associated with several adverse health outcomes, including carcinogenic effects, developmental issues in children, reproductive problems, and neurotoxicity. These compounds can interfere with the endocrine system, affecting hormones, and the immune system.
Dioxins and Furans: Dioxins and furans are unintended byproducts of industrial processes, such as waste incineration and chemical manufacturing. Their extreme toxicity and persistence in the environment have led to concerns about their health effects (3). Exposure to dioxins and furans can result in cancer, reproductive problems, developmental issues, and immune system dysfunction. These compounds are also known to disrupt the endocrine system, leading to hormonal imbalances.
PCDDs: Polychlorinated dibenzo-p-dioxins, or PCDDs, represent a subgroup of dioxins with extreme toxicity. Even at trace levels, PCDDs can have severe health effects, particularly on the liver. They have been linked to cancer, developmental issues, and immunological disorders. PCDDs, like other compounds in this group, can disrupt the endocrine system, affecting hormonal regulation.
The dangers of these chemicals are well-known. It’s impossible to completely remove them from our food chain, so the focus is on minimizing exposure to safe levels.
Dietary Sources
PCBs, Dioxins, Furans, and PCDDs in Fish: The seas and oceans serve as a reservoir for these toxic compounds. PCBs, dioxins, furans, and PCDDs enter the aquatic ecosystem through multiple pathways, including industrial discharges, incineration, and atmospheric deposition. Small aquatic organisms absorb these pollutants, which are then consumed by larger fish. This process continues up the food chain, with top-level predatory fish accumulating significant levels of these contaminants. Thus, fish, especially those higher in the food chain, serve as dietary sources of PCBs, dioxins, furans, and PCDDs.
Fish Oil and Cod Liver Oil: Fish oil and cod liver oil, both esteemed for their nutritional content, are concentrated sources of essential nutrients such as omega-3 fatty acids, and vitamins A and D. However, their fat content also means they can contain elevated levels of PCBs, dioxins, furans, and PCDDs. Cod liver oil, derived from the liver of codfish, can be of particular concern due to the liver’s role in detoxifying the fish’s body, potentially concentrating these toxic compounds.
Reporting & Regulation Of PCBs, Dioxins, Furans, And PCDD
Understanding Reporting
There are a large number of these chemicals, all of which have varying toxicity. Rather than reporting the amount of each individual chemical in foodstuffs, an aggregated toxic equivalent value (TEQ) is calculated. This calculation is a combination of the various quantities and toxic equivalent factor (TEF) of each compound. This makes testing and reporting easier to understand.
Typically, TEQ values are reported for a combination of PCDD and furans (PCDD/F-TEQ), or as a combination of PCDDs, furans, and PCBs (PCDD/F-PCB-TEQ).
EU And UK Regulation Of Environmental Toxins
The European Commission has set maximum thresholds for both PCDD/F-TEQ) and PCDD/F-PCB-TEQ in various foodstuffs. For fish oils, these maximum levels are:
- 1.75ng WHO-PCDD/ F-TEQ/Kg
- 6.0ng WHO-PCDD/F-PCB-TEQ/Kg (ref.)
These thresholds are set for foodstuffs to minimise the amount of these toxins humans accumulate based on typical dietary profiles, and available methods to remove them. Fish livers, for example, have a limit set at 20ng TEQ/Kg, which is significantly higher than fish oil, despite the fact that a typical portion of fish liver is much greater than a typical serving of marine oil.
USA Regulation Of Environmental Toxins
The USA recognises the toxicity of these chemicals and has taken steps to monitor and control consumption (e.g. some states have limits for water content). However, there is currently no legal limit for the content of fish oil.
Test Results
See below a graph of the test results for ARMORICA Fermented Cod Liver oil. The first result (green) shows the TEQ of crude fermented cod liver oil, at 28.21ng TEQ/kg. The following 4 samples show the development of our toxin-removal system, and the following results (blue) show the values of the products produced for retail. All results are significantly below the EU guidelines for fish oil.
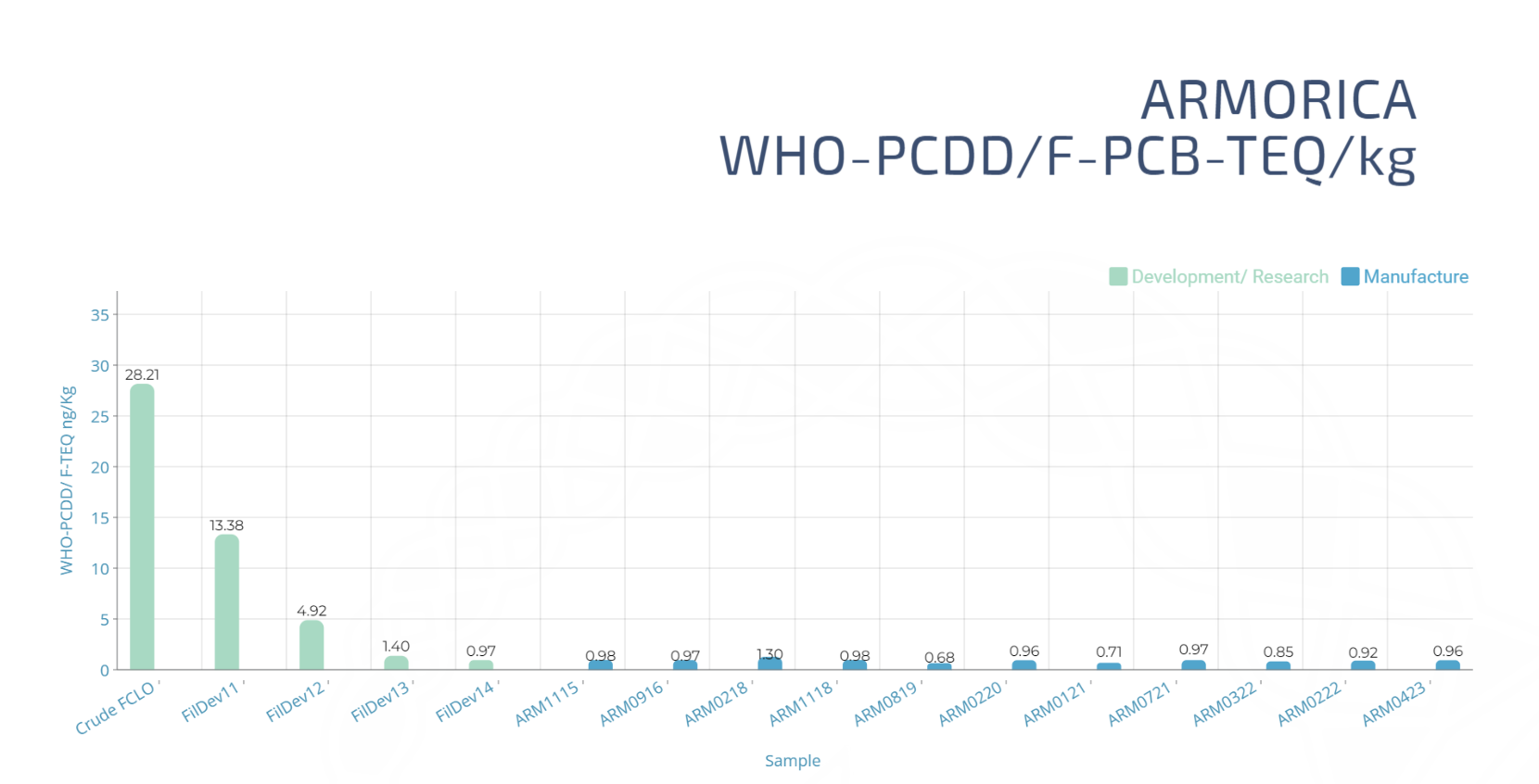
Comparison To Other Cod Liver Oils
Below is a graph showing the TEQ of 32 cod liver oils available to buy in the UK (red) compared with the average result of ARMORICA (blue), with a dotted line highlighting the 6ng TEQ/ Kg level.
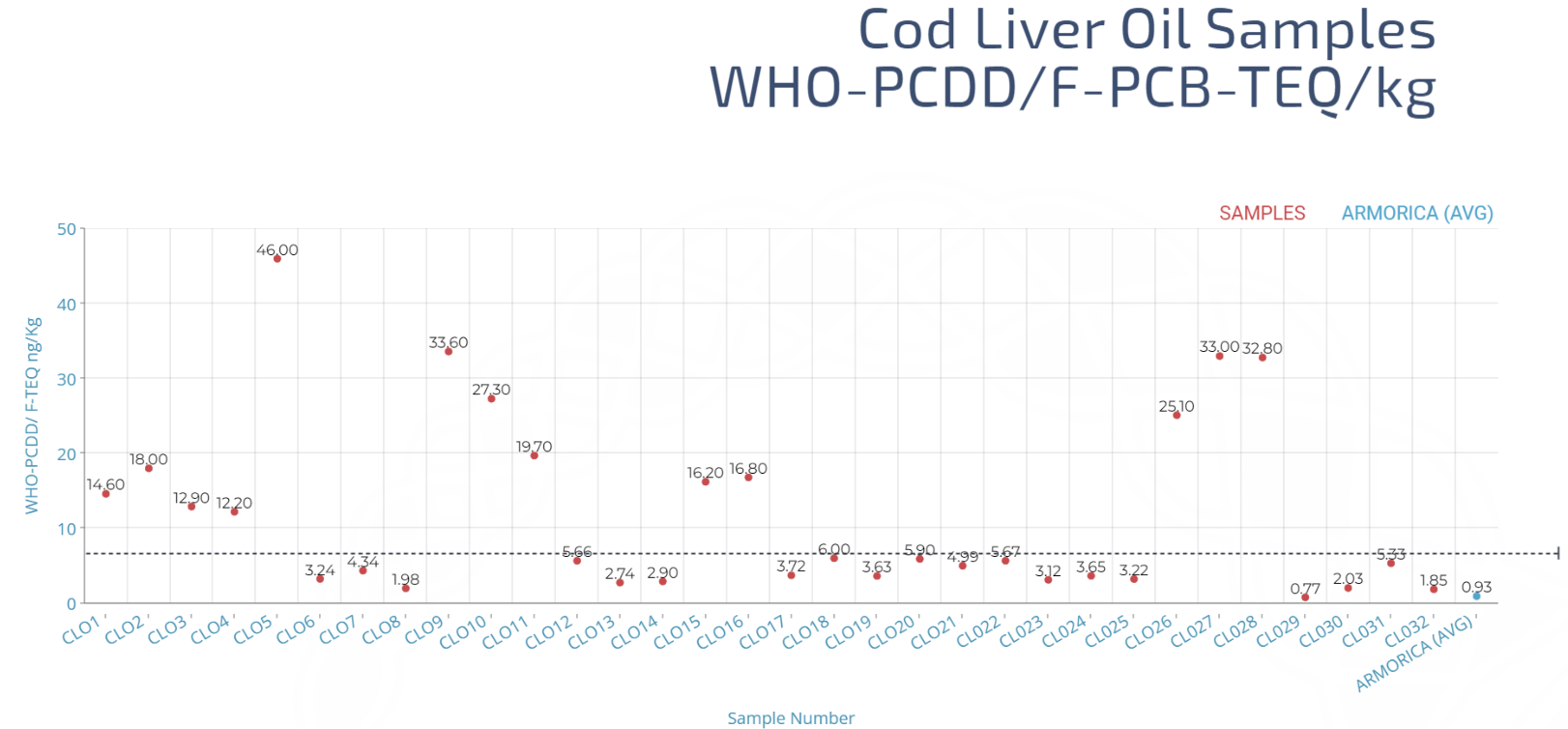
From the graph, you can see that 19 of the 32 samples taken have sufficiently low toxin levels, and 13 samples exceeded the limit. Of the 12 samples that meet EU requirements, only one sample had a lower TEQ than ARMORICA’s average.
Comparison To Other Food
As previously mentioned, these chemicals are a part of our food chain and are unavoidable. To put the above information into perspective, below is a table showing the TEQs of various meat products
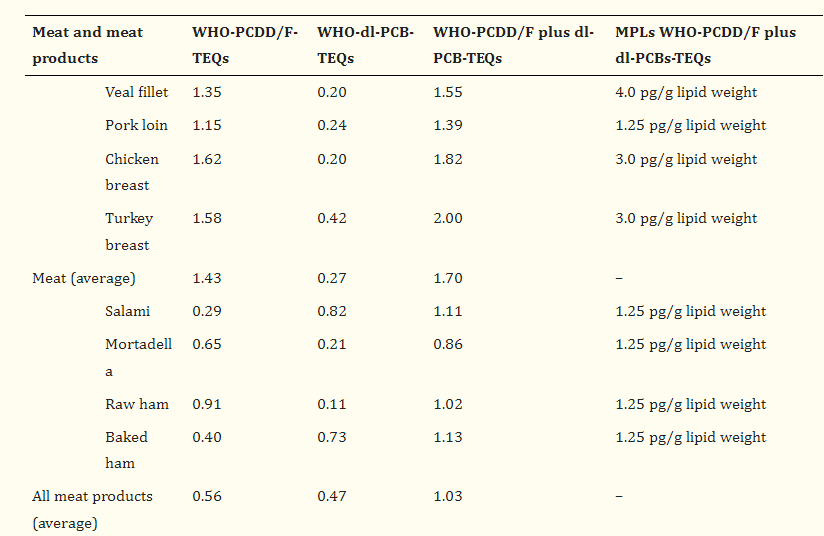
Typical meat products contain a higher TEQ than ARMORICA Fermented Cod Liver oil. Meat has an average of 1.7 TEQ/Kg, and meat products have an average of 1.03 TEQ/Kg, whereas ARMORICA Fermented cod liver oil has an average value of 0.93 TEQ/Kg. Additionally, you typically only consume 4g of cod liver oil daily, whereas meat fat can be several times that.
Summary
PCBs, Dioxins, Furans, and PCDD are harmful chemicals that bioaccumulate in the fats of animals and so are of particular concern in marine oils. The EU has set limits for the content of these toxins in marine oils to protect public health, which manufacturers are required to adhere to. Despite this, several brands of cod liver oil available in the UK do not meet these standards, and enforcement of these toxins is inconsistent.
The TEQ of ARMORICA’s cod liver oil is consistently below the limits set for cod liver oils and is comparable with typical meat products. The comparatively low amount of cod liver oil typically consumed compared to other animal fats further emphasises the safety of ARMORICA’s cod liver oil.
References
- Schecter A, et al. (2010). Dioxins, dibenzofurans, PCBs, and DDT in cod-liver oil from Norwegian and Swedish women: Determinants of the individual variations. Chemosphere, 80(2), 126-133.
- Hsu JF, et al. (2013). An overview of the health risks of dioxins on thyroid gland-related issues. International Journal of Environmental Research and Public Health, 10(10), 4544-4566.
- Hori T, et al. (2019). Dioxins, dioxin-like PCBs, non-dioxin-like PCBs, and PBDEs in commercial fish oil dietary supplements. Chemosphere, 234, 407-412.
- Lauby-Secretan B, et al. (2013). Carcinogenicity of polychlorinated biphenyls and polybrominated biphenyls. The Lancet Oncology, 14(4), 287-288.
- Schecter A, et al. (2010). Perfluorinated compounds, polychlorinated biphenyls, and organochlorine pesticide contamination in composite food samples from Dallas, Texas, USA. Environmental Health and Preventive Medicine, 15(6), 364-372.
- Van den Berg M, et al. (2013). The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicological Sciences, 93(2), 223-241.
- Schecter A, et al. (2011). Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environmental Health Perspectives, 119(10), 1517-1522.
- Mocarelli P, et al. (2008). Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environmental Health Perspectives, 116(1), 70-77.
- Focant JF, et al. (2013). Polybrominated diphenyl ethers and polychlorinated biphenyls in human adipose tissue from New York. Environment International, 59, 147-155.
- Malisch R, et al. (2011). Levels of polychlorinated dibenzo-p-dioxins, dibenzofurans, and biphenyls in human milk from different regions of Germany. Chemosphere, 85(10), 1675-1682.
- Schecter A, et al. (2006). Polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCD) in composite U.S. food samples. Environmental Health Perspectives, 114(10), 1515-1520.
- Institute of Medicine (US) Committee on the Implications of Dioxin in the Food Supply. Washington (DC): National Academies Press (US); 2003.
- Falandysz J, et al. (2019), A retrospective investigation into the occurrence and human exposure to polychlorinated naphthalenes (PCNs), dibenzo-p-dioxins and furans (PCDD/Fs) and PCBs through cod liver products (1972–2017). Chemosphere, 231 240-248